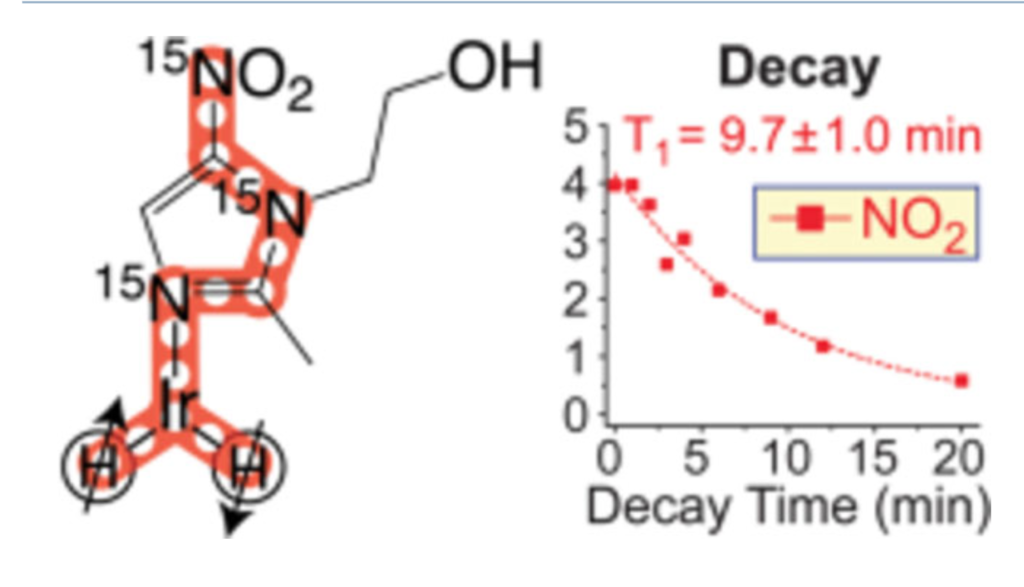
Hyperpolarization of 15N-labeled antibiotic metronidazole-15N3 is demonstrated using SABRE-SHEATH technique. A 15NO2 group is hyperpolarized via spin relays created by three 15N spins in metronidazole-15N3. In less than a minute of parahydrogen bubbling at ~0.4 μT, a high level of nuclear spin polarization P15N of ~16% is achieved on all three 15N sites at up to ~41 mM concentration. This product of 15N polarization and concentration of 15N spins is ~6-fold better than any previous value for 15N SABRE-derived hyperpolarization. At 1.4 T, T1 of the 15NO2 group is ~10 min. The large polarization and long T1 offer great potential for a wide range of in vivo metabolic applications from hypoxia sensing to theranostic imaging.
Bell LC et al, Tomography. 2019;5(1):110-117. doi: 10.18383/j.tom.2018.00041.
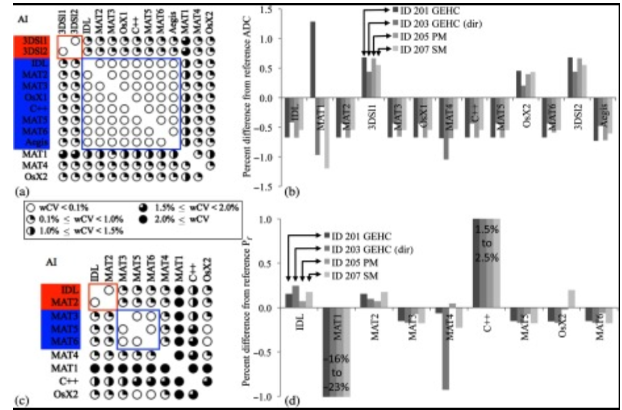
This study leveraged a dynamic susceptibility contrast MRI digital reference object to characterize rCBV consistency across 12 sites participating in the Quantitative Imaging Network (QIN), specifically focusing on differences in site-specific imaging protocols (IPs; n = 17), and PMs (n = 19) and differences due to site-specific IPs and PMs (n = 25). This study’s results have important implications for comparing rCBV values across sites and trails, where variability in PMs could confound the comparison of therapeutic effectiveness and/or any attempts to establish thresholds for categorical response to therapy.
Manhard MK et al, Magn Reson Med. 2019;82(3):973-983. doi: 10.1002/mrm.27784. Epub 2019 May 8.
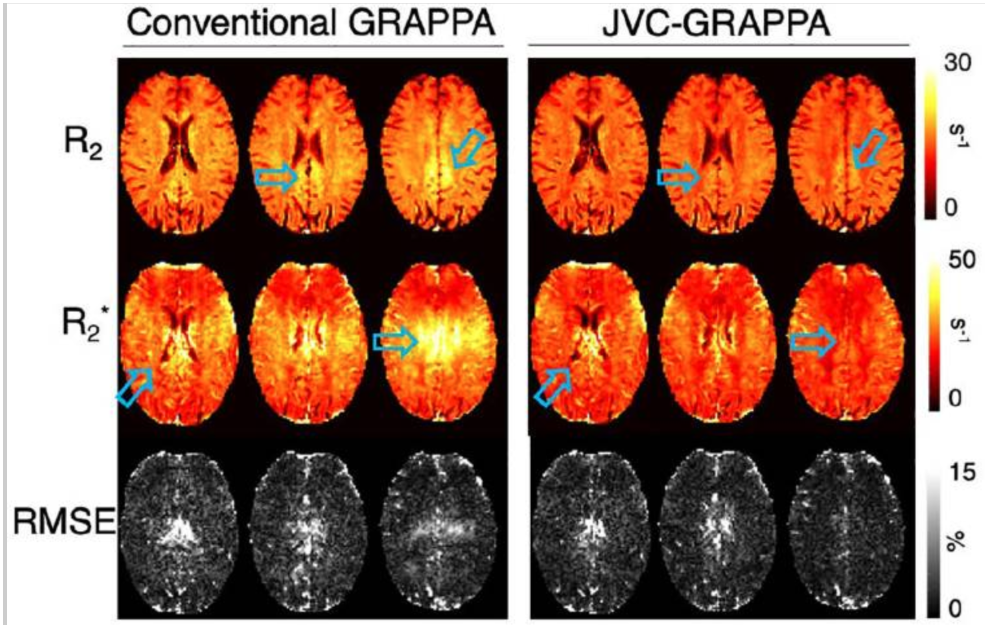
We present an accelerated sequence that provides whole-brain coverage at an improved spatio-temporal resolution, to allow for dynamic quantitative R2 and R2 * mapping during contrast-enhanced imaging. A multi-echo spin and gradient-echo sequence were implemented with simultaneous multislice acquisition. Results showed improved quantitative maps with a 9-fold acceleration factor and whole-brain coverage during the dynamic perfusion acquisition. This method shows the advantage of using a joint virtual coil-GRAPPA reconstruction to allow for high acceleration factors while maintaining reliable image quality for quantitative perfusion mapping.
Schmainda KM et al, AJNR Am J Neuroradiol. 2018;39(6):1008-1016. doi: 10.3174/ajnr.A5675.
This study compares multisite/multiplatform analyses of shared DSC-MR imaging datasets of patients with brain tumors. Data was collected after a preload and during a bolus injection of gadolinium contrast agent using a gradient recalled-echo-EPI sequence, and included a predetermined arterial input function; enhancing tumor ROIs and ROIs necessary to create normalized relative CBV and CBF maps. Results showed that for normalized relative CBV and CBF, 93% and 94% of entries showed good or excellent cross-site agreement. Therefore, by means of DSC-MR imaging data obtained after a preload of contrast agent, substantial consistency resulted across sites for brain tumor perfusion metrics with a common threshold discoverable for distinguishing low- from high-grade tumors.
Newitt D et al, J Med Imaging. 2018;5(1):011003. doi: 10.1117/1.JMI.5.1.011003.
We examined the variability in the apparent diffusion coefficient (ADC) obtained from both post-processing software implementations utilized by the NCI Quantitative Imaging Network and online scan time-generated ADC maps. Concordance of the majority of implementations was excellent for both phantom ADC measures and in vivo, with relative biases, and phantom but with higher deviations in ADC at the lowest phantom ADC values. In vivoconcordance was good, with typical biases of 3% but higher for online maps. Multiple b-value ADC implementations were separated into two groups determined by the fitting algorithm. Intergroup mean ADC differences ranged from negligible for phantom data to 2.8% for in vivo data. Some higher deviations were found for individual implementations and online parametric maps. Despite generally good concordance, implementation biases in ADC measures are sometimes significant and may be large enough to be of concern in multisite studies.
Gale EM et al, Radiology. 2018; 286(3):865-872. doi: 10.1148/radiol.2017170977
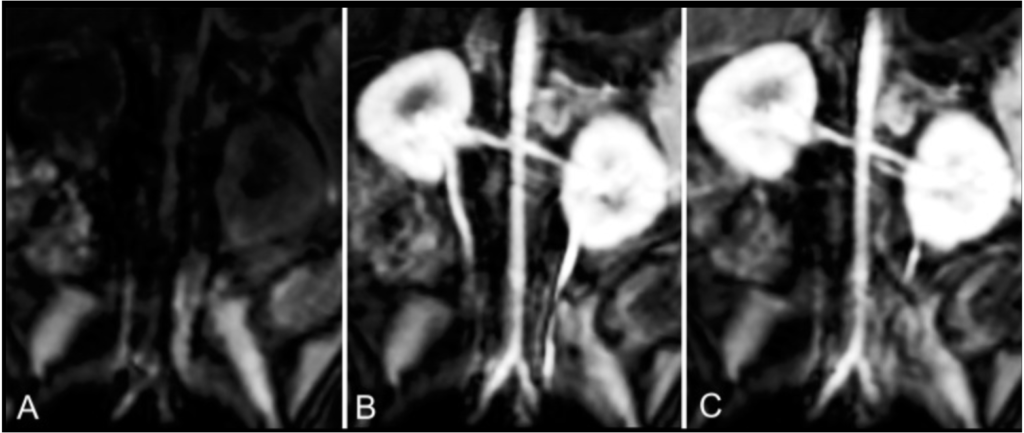
This study compared intravascular contrast enhancement produced by the manganese-based magnetic resonance (MR) imaging contrast agent manganese-N-picolyl-N,N’,N’-trans-1,2-cyclohexenediaminetriacetate (Mn-PyC3A) to gadopentetate dimeglumine (Gd-DTPA) and evaluated the excretion, pharmacokinetics, and metabolism of Mn-PyC3A. Contrasted material-enhanced MR angiography was performed in baboons using Mn-PyC3A and Gd-DTPA. Data revealed that Mn-PyC3A enables contrast-enhanced MR angiography with comparable contrast enhancement to gadolinium-based agents and may overcome concerns regarding gadolinium-associated toxicity and retention.
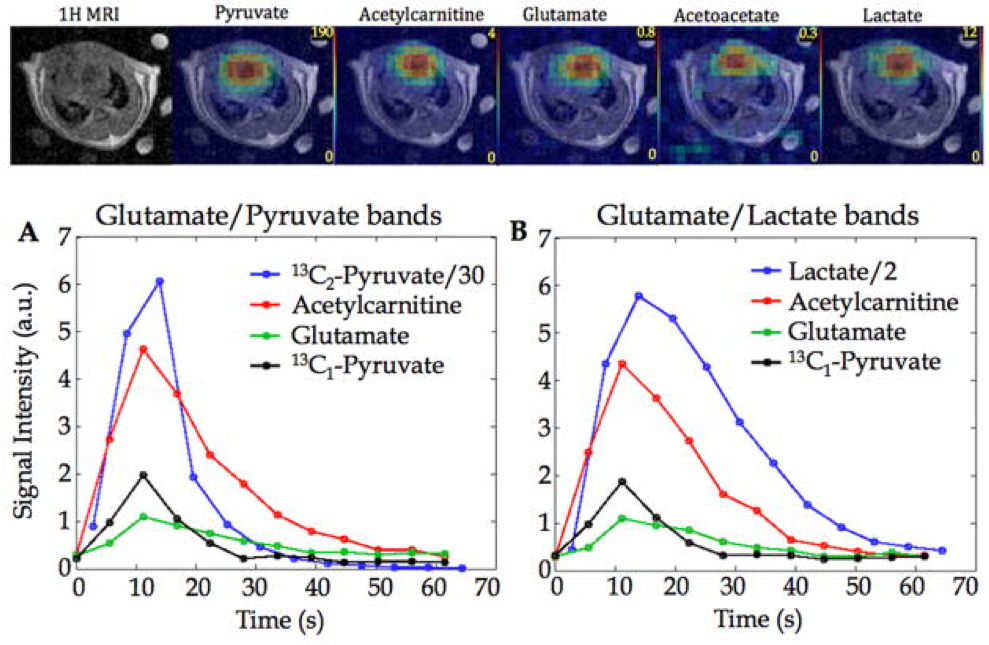
Hyperpolarized [2-13C] pyruvate permits the ability to follow the 13C label beyond flux through pyruvate dehydrogenase complex and investigate the incorporation of acetyl-CoA into different metabolic pathways. However, chemical shift imaging (CSI) with [2-13C] pyruvate is challenging due to the large spectral dispersion of the resonances. In this work, we presented a method that allows CSI of widely separated resonances without chemical shift displacement artifact, acquiring multiple frequency bands alternately to obtain dynamic time-course information. This approach enables robust imaging of downstream metabolic products of acetyl-CoA with hyperpolarized [2-13C] pyruvate.
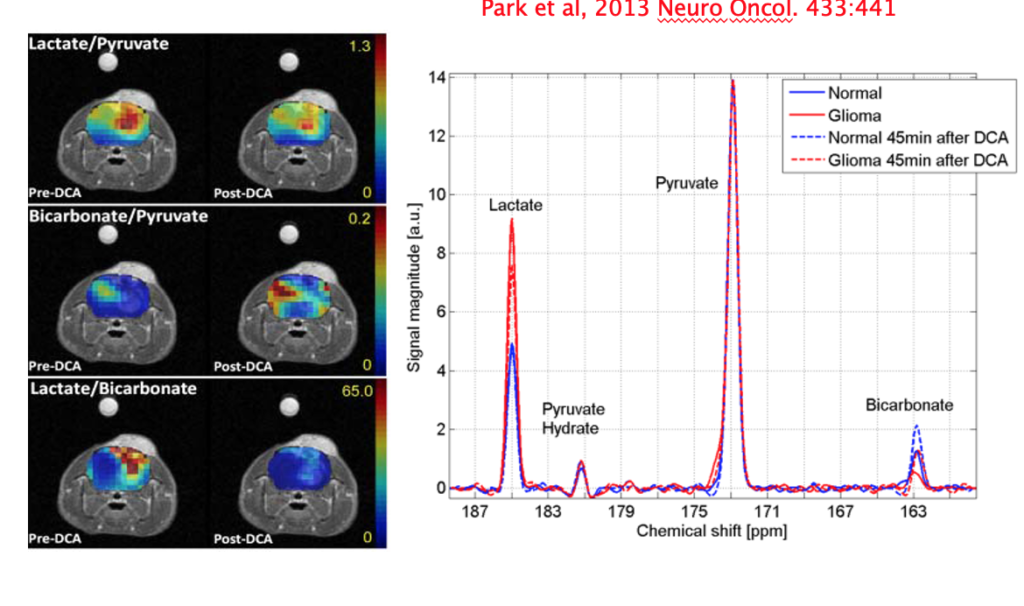
The metabolic phenotype that derives disproportionate energy via glycolysis in solid tumors, including glioma, leads to elevated lactate labeling in metabolic imaging using hyperpolarized [1-13C]pyruvate. Although the pyruvate dehydrogenase (PDH)–mediated flux from pyruvate to acetyl coenzyme A can be indirectly measured through the detection of carbon-13 (13C)-labeled bicarbonate, it has proven difficult to visualize 13C-bicarbonate at high enough levels from injected [1-13C]pyruvate for quantitative analysis in brain. The aim of this study is to improve the detection of 13C-labeled metabolites, in particular bicarbonate, in glioma and normal brain in vivo and to measure the metabolic response to dichloroacetate, which upregulates PDH activity.
Bane O et al. Magn Reson Med. 2018;79(5):2564-2575. doi: 10.1002/mrm.26903.
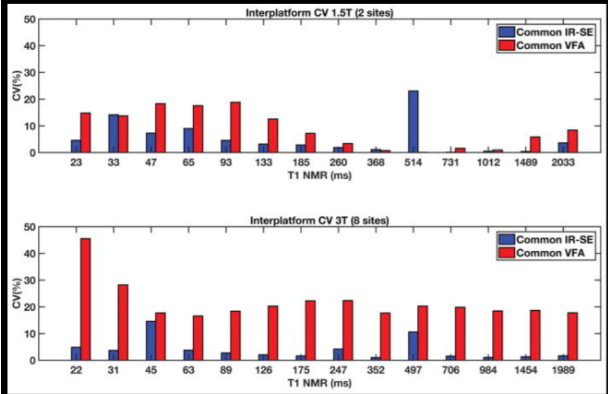
The purpose of this study was to determine the in vitro accuracy, test-retest repeatability, and interplatform reproducibility of T1 quantification protocols used for dynamic contrast-enhanced MRI at 1.5 and 3 T. A T1 phantom with 14 samples was imaged at eight centers with a common inversion-recovery spin-echo (IR-SE) protocol, and a variable flip angle (VFA) protocol. Results showed the common IR-SE protocol, accuracy was influenced predominantly by T1 sample , whereas test-retest repeatability was influenced by the scanner. Among the site-specific protocols, Look-Locker inversion recovery and VFA protocols showed the best accuracy and repeatability. The VFA protocols with 2 to 3 flip angles achieved acceptable balance of extensive spatial coverage, accuracy, and repeatability in T1 quantification. However, further optimization of flip-angle choice for each tissue application, and B1 correction, are needed to improve the robustness of VFA protocols for T1 mapping.
Josan S et al. NMR Biomed. 2013;26(12):1680-1687.
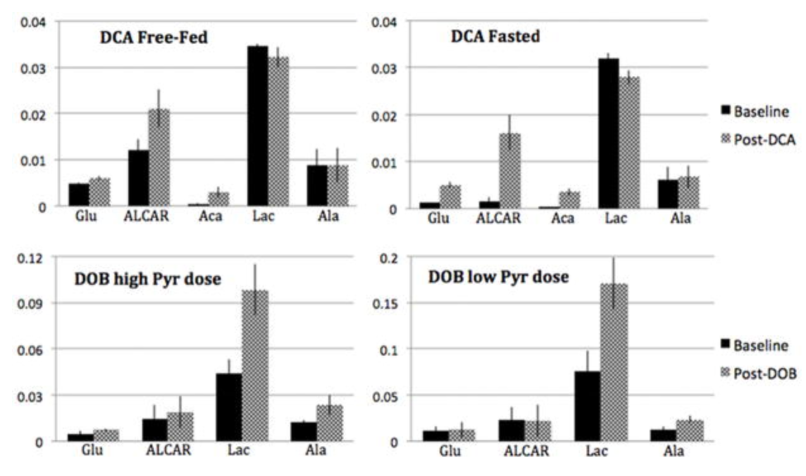
This work investigates changes in the metabolic fate of acetyl-CoA in response to metabolic interventions of dichloroacetate (DCA)-induced increased pyruvate dehydrogenase complex (PDC) flux in the fed and fasted state, and increased cardiac workload with dobutamine in vivo in rat heart at two different pyruvate doses. DCA led to a modest increase in the 13C labeling of [5-13C]glutamate, and a considerable increase in [1-13C]acetylcarnitine and [1,3-13C]acetoacetate peaks. Dobutamine resulted in an increased labeling of [2-13C]lactate, [2-13C]alanine and [5-13C]glutamate. The relative changes in the different metabolic products provide information about the relationship between PDC-mediated oxidation of pyruvate and its subsequent incorporation into the TCA cycle compared to other metabolic pathways.
Josan S et al. NMR Biomed. 2013;26(6):607-612.
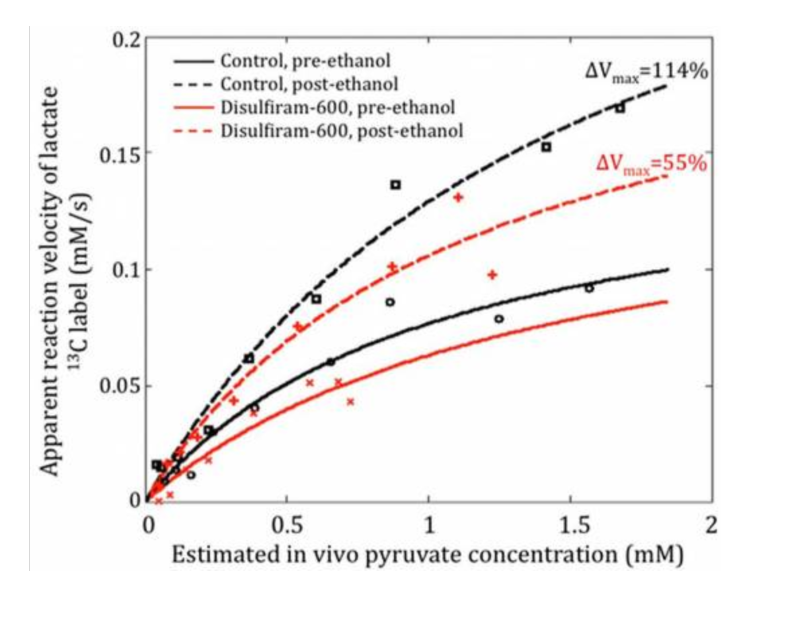
Aldehyde dehydrogenase-2 (ALDH2), is a critical mitochondrial enzyme for eliminating certain cytotoxic aldehydes in the body and a promising target for drug development. In this work, we show that dynamic magnetic resonance spectroscopic imaging of hyperpolarized [1-13C]pyruvate and its conversion to [1-13C]lactate can provide an indirect in vivo measurement of ALDH2 activity via the concentration of NADH. Results from a rat liver ethanol model (n = 9) show that changes in 13C-lactate labeling following the bolus injection of hyperpolarized pyruvate are highly correlated with changes in ALDH2 activity (R2=0.76).
Exchange-linked dissolution agents in dissolution-DNP (13) C metabolic imaging
Hurd RE et al. Magn Reson Med. 2013;70:936–942.
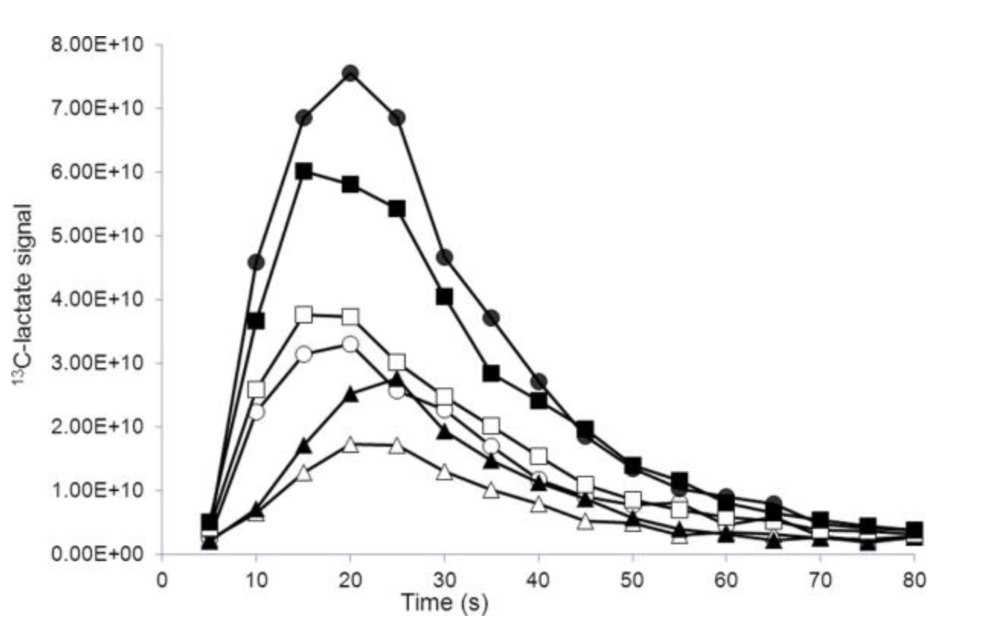
The use of unlabeled exchange-linked dissolution agents (ELDA) in hyperpolarized metabolic imaging was studied to examine pool size limits and saturation relative to the availability of NADH. 3-D dynamic metabolic images were obtained and comparisons were made on the basis of apparent rate constants and [1-13C]lactate signal-to-noise ratio. Isotope exchange was also probed in the reverse direction. Results showed that liver, kidney and vascular regions of interest increased in [1-13C]lactate signal with added unlabeled sodium lactate in the dissolution buffer. These results are consistent with a high level of 13C-exchange, and with labeling rates that are limited by steady-state pool sizes in vivo.
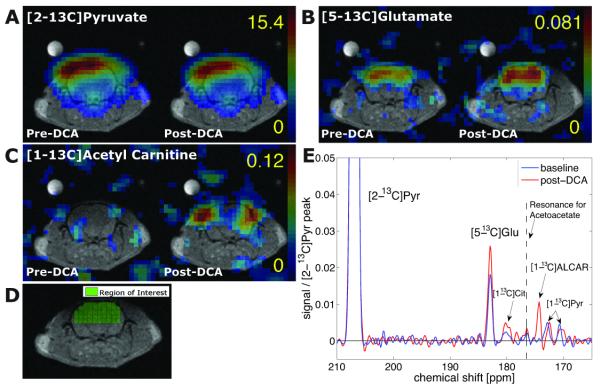
Hyperpolarized [1-(13) C]pyruvate ([1-(13) C]Pyr) has been used to assess metabolism in healthy and diseased states, focusing on the downstream labeling of lactate (Lac), bicarbonate and alanine. Although hyperpolarized [2-(13) C]Pyr, which retains the labeled carbon when Pyr is converted to acetyl-coenzyme A, has been used successfully to assess mitochondrial metabolism in the heart, the application of [2-(13) C]Pyr in the study of brain metabolism has been limited to date, with Lac being the only downstream metabolic product reported previously. In this study, single-time-point chemical shift imaging data were acquired from rat brain in vivo. [5-(13) C]Glutamate, [1-(13) C]acetylcarnitine and [1-(13) C]citrate were detected in addition to resonances from [2-(13) C]Pyr and [2-(13) C]Lac. Brain metabolism was further investigated by infusing dichloroacetate, which upregulates Pyr flux to acetyl-coenzyme A. After dichloroacetate administration, a 40% increase in [5-(13) C]glutamate from 0.014 ± 0.004 to 0.020 ± 0.006 (p = 0.02), primarily from brain, and a trend to higher citrate (0.002 ± 0.001 to 0.004 ± 0.002) were detected, whereas [1-(13) C]acetylcarnitine was increased in peripheral tissues. This study demonstrates, for the first time, that hyperpolarized [2-(13) C]Pyr can be used for the in vivo investigation of mitochondrial function and tricarboxylic acid cycle metabolism in brain.
This article describes the basic physics of dissolution dynamic nuclear polarization (dissolution‐DNP), and the impact of the resulting highly nonequilibrium spin states, on the physics of magnetic resonance imaging (MRI) detection. The hardware requirements for clinical translation of this technology are also presented. For studies that allow the use of externally administered agents, hyperpolarization offers a way to overcome normal magnetic resonance sensitivity limitations, at least for a brief T1‐dependent observation window. A 10,000–100,000‐fold signal‐to‐noise advantage provides an avenue for real‐time measurement of perfusion, metabolite transport, exchange, and metabolism. The principles behind these measurements, as well as the choice of agent, and progress toward the application of hyperpolarized 13C metabolic imaging in oncology, cardiology, and neurology are reviewed.
We report metabolic images of 13C, following injection of a bolus of of hyperpolarized [1-13C] pyruvate in a live rat. The data were acquired on a clinical scanner, using custom coils for volume transmission and array reception. Proton blocking of all carbon resonators enabled proton anatomic imaging with the system body coil, to allow for registration of anatomic and metabolic images, for which good correlation was achieved, with some anatomic features (kidney and heart) clearly visible in a carbon image, without reference to the corresponding proton image. Parallel imaging with sensitivity encoding was used to increase the spatial resolution in the SI direction of the rat. The signal to noise ratio in was in some instances unexpectedly high in the parallel images; variability of the polarization among different trials, plus partial volume effects, are noted as a possible cause of this
MacKenzie JD, et al. Radiology. 2011;259(2):414-420.
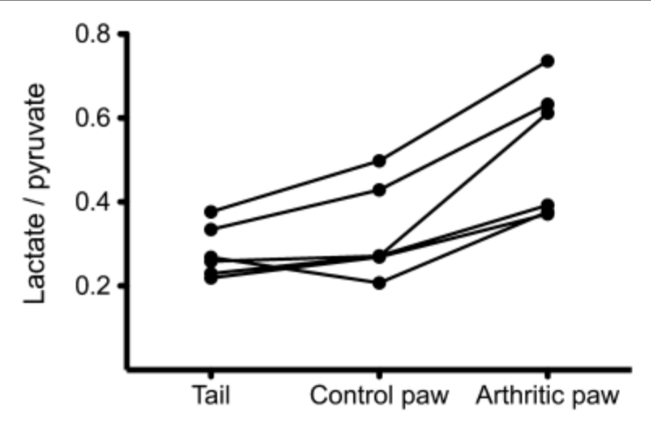
In this study we examined the feasibility of using magnetic resonance (MR) spectroscopy with hyperpolarized carbon 13 ((13)C)-labeled pyruvate to detect inflammation. We induced arthritis into the right hind paw of six rats; the left hind paw served as an internal control. The lactate dehydrogenase-catalyzed conversion of pyruvate to lactate was then measured in the inflamed and control paws using (13)C MR spectroscopy. Hyperpolarized (13)C-pyruvate was intravenously injected into the rats before simultaneous imaging. All animals showed findings of inflammation in the affected paws and no signs of arthritis in the control paws. These results suggest that alterations in the conversion of pyruvate to lactate as detected with (13)C-MR spectroscopy may be indicative of the presence of inflammatory arthritis.
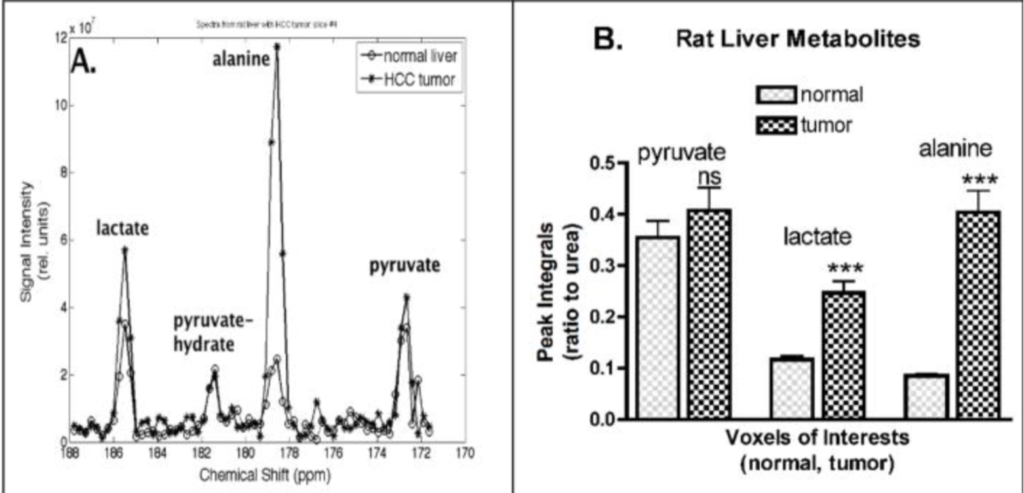
In vivo MRSI of hyperpolarized [1-(13)C]pyruvate metabolism in rat hepatocellular carcinoma
Darpolor MM, et al. NMR Biomed. 2011;24(5):506-513.
Hepatocellular carcinoma (HCC) is the primary form of human adult liver malignancy. Reportedly, high levesl of [1-(13)C]alanine are found in an orthotopic HCC. In this study we implemented a 3D MRSI sequence to investigate the potential hallmark of cellular metabolism in rat livers bearing HCC (n = 7 buffalo rats). In this experiment, enzyme levels were significantly higher in tumor than in normal liver tissues within each rat, and were associated with the in vivo MRSI signal of [1-(13)C]alanine and [1-(13)C]lactate after an injection of [1-(13)C]pyruvate. Histopathological analysis of these tumors confirmed the successful growth of HCC as a nodule in buffalo rat livers, revealing malignancy and hypervascular architecture. The results also demonstrated that the metabolic fate of pyruvate conversion to alanine significantly superseded that of pyruvate conversion to lactate, potentially serving as a marker of HCC tumors.
Josan S, et al. J Magn Reson. 2011;209(2):332-336.
Undersampled spiral CSI (spCSI) using a free induction decay (FID) acquisition allows sub-second metabolic imaging of hyperpolarized ¹³C. Phase correction of the FID acquisition can be difficult, especially with contributions from aliased out-of-phase peaks. This work extends the spCSI sequence by incorporating double spin echo radiofrequency (RF) pulses to eliminate the need for phase correction and obtain high quality spectra in magnitude mode. The sequence also provides an added benefit of attenuating signal from flowing spins, which can otherwise contaminate signals in the organ of interest. However, care must be taken for dynamic imaging to ensure that the spins remain within the B₁-homogeneous sensitive volume of the RF coil.
Mayer D, et al. Magn Reson Med. 2011;65(5):1228-1233.
Fast chemical shift imaging (CSI) techniques are advantageous in metabolic imaging of hyperpolarized compounds due to the limited duration of the signal amplification. Here a high-performance gradient insert was used coupled with undersampled spiral CSI to increase the imaging speed or the spatial resolution of hyperpolarized (13)C metabolic imaging. Both a single-shot sequence with a total acquisition time of 125 ms and a three-shot sequence with a nominal in-plane resolution of 1.5 mm were implemented. The technique was applied to metabolic imaging of the rat brain in vivo after the injection of hyperpolarized [1-(13)C]-pyruvate. Dynamic imaging afforded the measurement of region-of-interest-specific time courses of pyruvate and its metabolic products, while imaging at high spatial resolution was used to better characterize the spatial distribution of the metabolite signals.
Xu T, et al. NMR Biomed. 2011;24(8):997-1005.
In this work, we developed an efficient quantification framework employing a spiral-based dynamic spectroscopic imaging approach. The approach overcomes the aforementioned limitations and demonstrates that the in vivo (13)C labeling of lactate and alanine after a bolus injection of [1-(13)C]pyruvate is well approximated by saturatable kinetics, and apparent Michaelis constant K(M) being unbiased with respect to critical experimental parameters. We focused on the (13)C labeling of lactate and alanine and did not differentiate the labeling mechanism or the respective contribution of various factors to the labeling process.
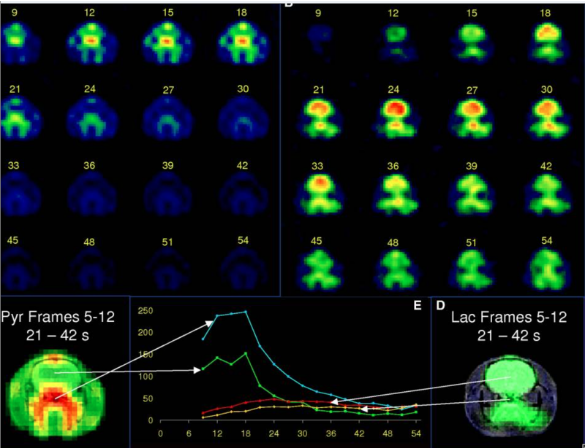
Hurd RE et al. J Cereb Blood Flow Metab. 2010;30(10):1734-1741.
Dynamic hyperpolarized [1-13C]pyruvate metabolic imaging in the normal anesthetized rat brain is demonstrated on a clinical 3-T magnetic resonance imaging scanner. A 12-second bolus injection of hyperpolarized [1-13C]pyruvate is imaged at a 3-second temporal resolution. The observed dynamics are evaluated with regard to cerebral blood volume (CBV), flow, transport, and metabolic exchange with the cerebral lactate pool. A model for brain [1-13C]lactate, based on blood–brain transport kinetics, CBV, and the observed pyruvate dynamics is described.
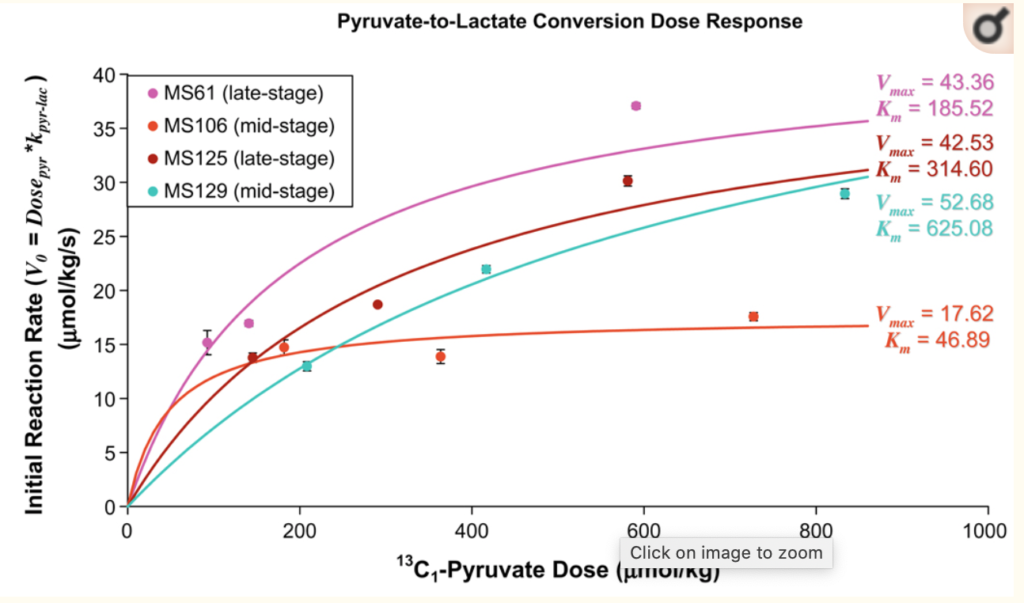
The purpose of the study was to investigate metabolic exchange between 13C1-pyruvate, 13C1-lactate, and 13C1-alanine in preclinical model systems using kinetic modeling of dynamic hyperpolarized 13C spectroscopic data and to examine the relationship between fitted parameters and dose–response.
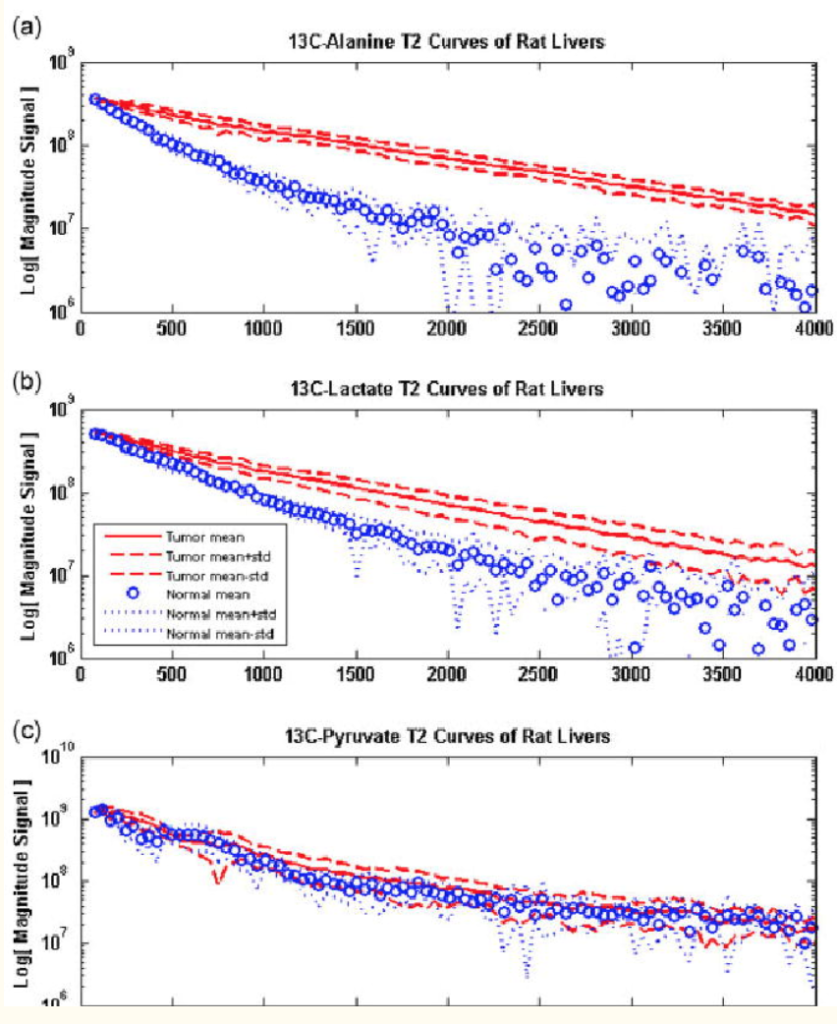
NMR hyperpolarization of uniformly 15N-labeled metronidazole-15N3 is demonstrated using SABRE-SHEATH (Signal Amplification by Reversible Exchange in SHield Enables Alignment Transfer to Heteronuclei). In this antibiotic, a 15NO2 group is hyperpolarized via spin relays created by 15N spins in metronidazole-15N3, and the polarization is transferred from parahydrogen-derived hydrides over six chemical bonds. This efficient spin-relayed mechanism of polarization transfer is supported by measurements of polarization dynamics and T1 relaxation in micro-Tesla magnetic fields. In less than a minute of parahydrogen bubbling at ~0.4 μT, a high level of nuclear spin polarization P15N of ~16% is achieved on all three 15N sites of metronidazole-15N3 at up to ~41 mM concentration. This product of 15N polarization and concentration of 15N spins is ~6-fold better than any previous value for 15N SABRE-derived hyperpolarization. At 1.4 T, the hyperpolarized state of the 15NO2 group of this antibiotic persists for tens of minutes (T1~10 min), in part because its 15N spin has no detectable spin-spin couplings with protons of the molecule. A novel synthesis achieving robust uniform 15N enrichment of metronidazole is reported with 15% yield over three steps. This synthetic approach should be suitable for preparation of other 15N-labeled nitroimidazole derivatives, potentially enabling a wide range of in vivo metabolic probes from hypoxia sensing to theranostic imaging.
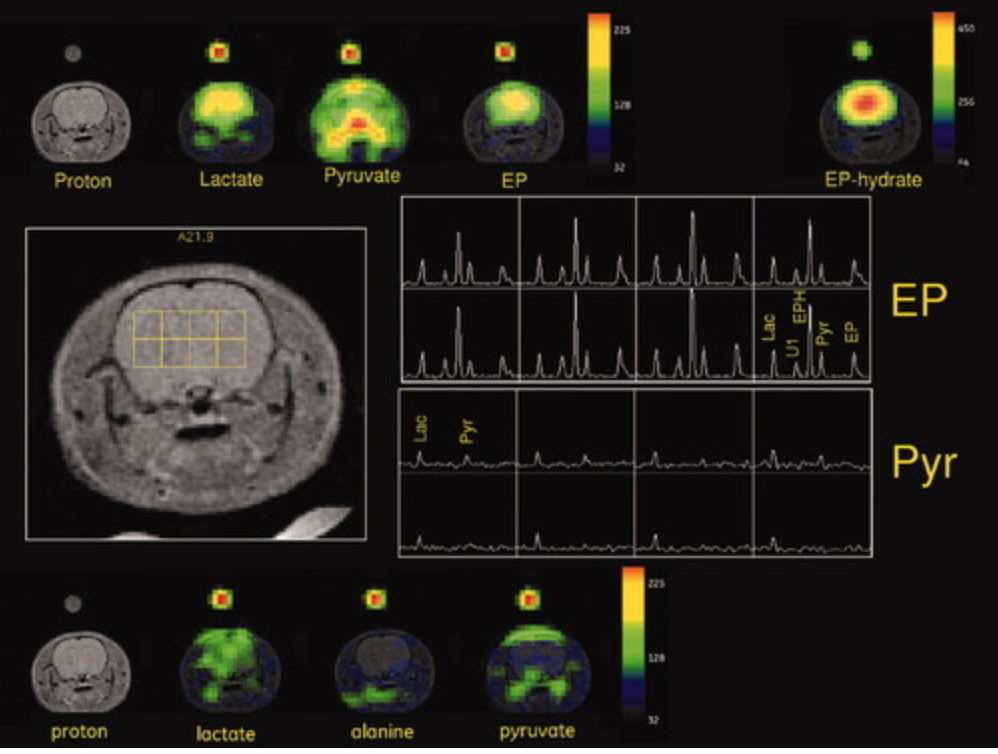
Formulation, polarization, and dissolution conditions were developed to obtain a stable hyperpolarized solution of [1‐13C]‐ethyl pyruvate. A maximum tolerated concentration and injection rate were determined, and 13C spectroscopic imaging was used to compare the uptake of hyperpolarized [1‐13C]‐ethyl pyruvate relative to hyperpolarized [1‐13C]‐pyruvate into anesthetized rat brain. Hyperpolarized [1‐13C]‐ethyl pyruvate and [1‐13C]‐pyruvate metabolic imaging in normal brain is demonstrated and quantified in this feasibility and range‐finding study.
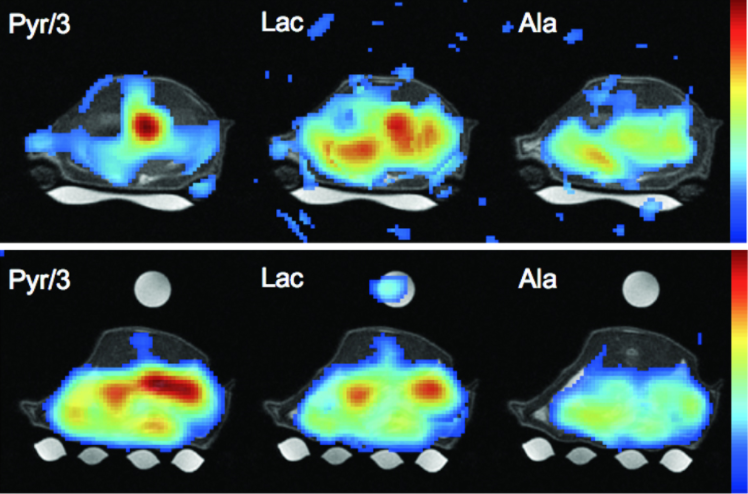
Dynamic nuclear polarization can create hyperpolarized compounds with MR signal‐to‐noise ratio enhancements on the order of 10,000‐fold. Both exogenous and normally occurring endogenous compounds can be polarized, and their initial concentration and downstream metabolic products can be assessed using MR spectroscopy. Given the transient nature of the hyperpolarized signal enhancement, fast imaging techniques are a critical requirement for real‐time metabolic imaging. We report on the development of an ultrafast, multislice, spiral chemical shift imaging sequence, with subsecond acquisition time, achieved on a clinical MR scanner. The technique was used for dynamic metabolic imaging in rats, with measurement of time‐resolved spatial distributions of hyperpolarized 13C1‐pyruvate and metabolic products 13C1‐lactate and 13C1‐alanine, with a temporal resolution of as fast as 1 s. Metabolic imaging revealed different signal time courses in liver from kidney. These results demonstrate the feasibility of real‐time, hyperpolarized metabolic imaging and highlight its potential in assessing organ‐specific kinetic parameters.
Costa AF et al. Magn Reson Imaging. 2010;28(2):185-194.
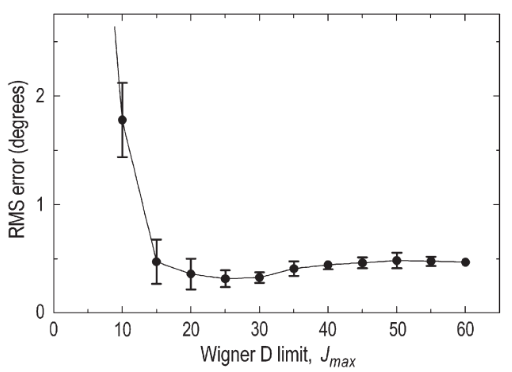
Subject motion remains a challenging problem to overcome in clinical and research applications of magnetic resonance imaging (MRI). Subject motion degrades the quality of MR images and the integrity of experimental data. A promising method to correct for subject motion in MRI is the spherical navigator (SNAV) echo. In this study, we assessed the accuracy, precision and computational requirements of measuring rotations about all three coordinate axes by correlating the spherical harmonic expansions of SNAV data. We compare the results of this technique to previous SNAV studies and found that the spherical harmonic technique is a highly accurate method to register SNAVs and detect 3D rotations in MRI. This registration technique is currently well suited for retrospective motion correction applications, such as removing motion-related image artifacts and aligning slices within a high-resolution 3D volume.
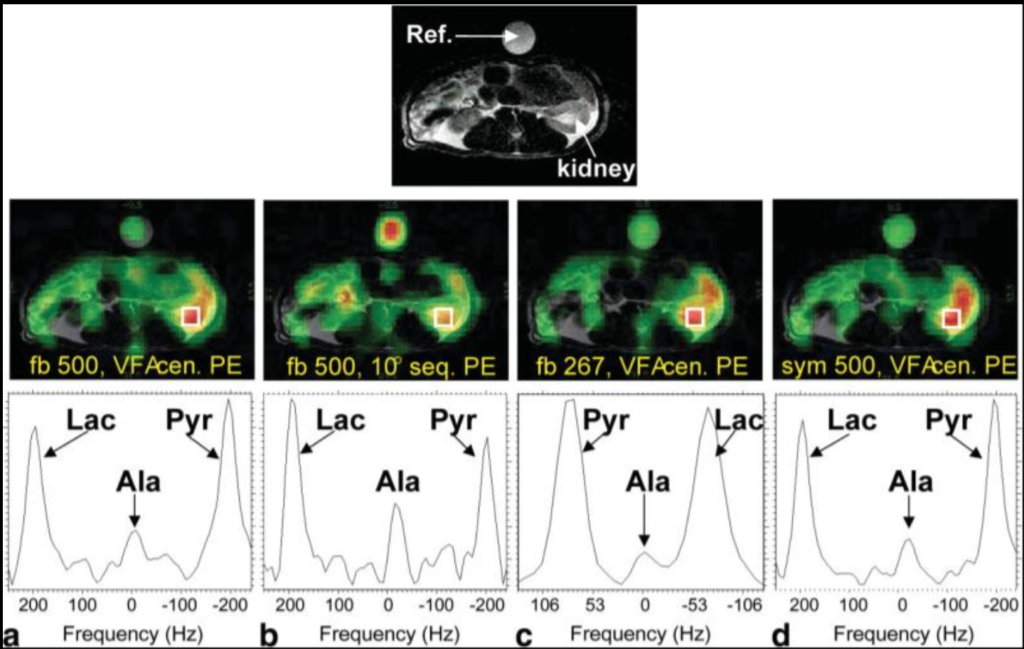
Imaging considerations for in vivo 13C metabolic mapping using hyperpolarized 13C-pyruvate.
Yen YF et al. Magn Reson Med. 2009;62(1):1-10.
One of the challenges of optimizing signal-to-noise ratio (SNR) and image quality in (13)C metabolic imaging using hyperpolarized (13)C-pyruvate is the different MR signal time-courses for pyruvate and its metabolic products. The image timing was set to coincide with the peak production of lactate. In addition, two aspects of EPSI sampling strategies were explored: waveform design (flyback vs. symmetric EPSI) and spectral bandwidth (BW = 500 Hz vs. 267 Hz). Both symmetric EPSI and reduced BW trended toward increased SNR. The imaging strategies reported here can serve as guidance to other multishot spectroscopic imaging protocols for (13)C metabolic imaging applications.
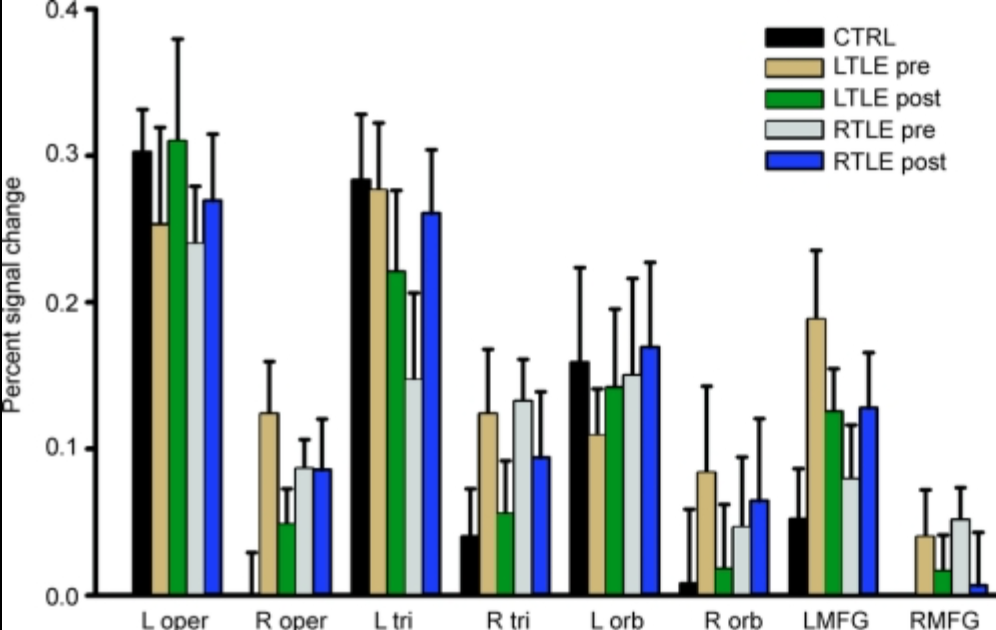
Wong SW et al. Neurology. 2009;73(7):518-525.
Functional MRI was used to study the impact of temporal lobe epilepsy (TLE) and anterior temporal lobectomy (ATL) on the cortical language network in patients with medically refractory TLE. Nineteen patients with medically refractory TLE and 11 healthy control subjects were enrolled in this study. 10 underwent left ATL, and 9 underwent right ATL. The subjects silently generated verbs in response to a series of visually presented nouns inside the scanner. The patients with TLE showed increased neural activity in the right inferior frontal gyri (IFG) and middle frontal gyri (MFG). The right TLE patients demonstrated strong correlation between their language performance and the level of cortical activation within the typical language areas. After the ATL surgery, the left TLE patients showed reduced activation in the left MFG and right IFG, whereas no difference was observed in the right TLE patients. Therefore, this study demonstrates that the cortical language network is affected differently by the left and right temporal lobe epilepsy and is reorganized after anterior temporal lobectomy.
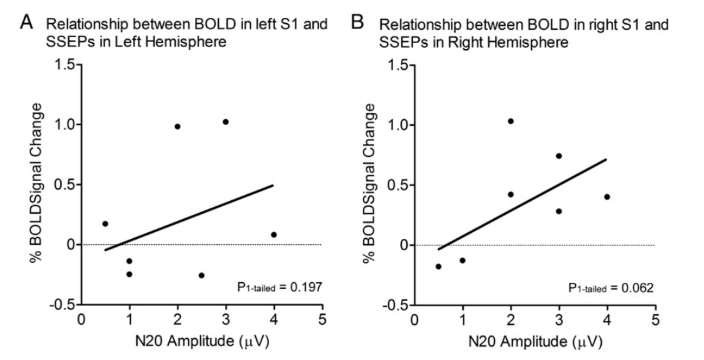
Functional MRI study of the primary somatosensory cortex in comatose survivors of cardiac arrest.
Gofton TE et al. Exp Neurol. 2009;217(2):320-327.
Although it is difficult to assess cerebral function in comatose patients, fMRI of cardiac arrest patients could provide further insight into cerebral function during coma. Using fMRI, cerebral activation to somatosensory stimulation to the palm of the hand was measured in 19 comatose survivors of cardiac arrest and in 10 healthy control subjects. Five out of 19 patients were alive at 3 months. Patients who survived cardiac arrest showed greater BOLD in S1 contralateral to somatosensory stimulation of the hand compared to patients who eventually did not. Greater BOLD was also seen in S1 of patients who retained their SSEP N20 waveforms, and positive correlations between BOLD in S1 with both levels of consciousness and measures of outcome at 3 months. In summary, this study demonstrates that BOLD in the S1 contralateral to somatosensory stimulation of the hand varies with clinical measures of the level of consciousness during coma.
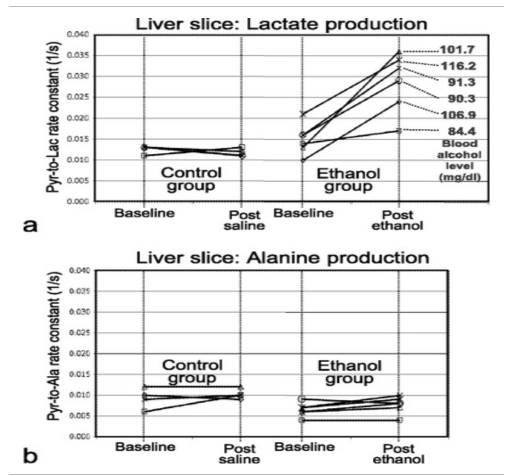
Spielman DM et al. Magn Reson Med. 2009;62(2):307-313.
In this work (13)C-MRS of hyperpolarized [1-(13)C]pyruvate was used to interrogate a metabolic pathway involved in neither aerobic nor anaerobic metabolism. Excess ethanol consumption leads to altered liver metabolism, which is associated with adverse medical conditions including fatty liver disease, hepatitis, cirrhosis, and cancer. Here we present a method for noninvasively monitoring this important process in vivo. After a bolus injection of hyperpolarized [1-(13)C]pyruvate, a significantly increased rat liver lactate production rate was observed. The effect is attributable to increased liver nicotinamide adenine dinucleotide (NADH). Beyond studies of liver metabolism, this novel in vivo assay of changes in NADH levels makes hyperpolarized [1-(13)C]pyruvate a potentially viable substrate for studying the multiple in vivo metabolic pathways that use NADH (or NAD(+)) as a coenzyme, thus broadening the range of applications that have been discussed in the literature to date.
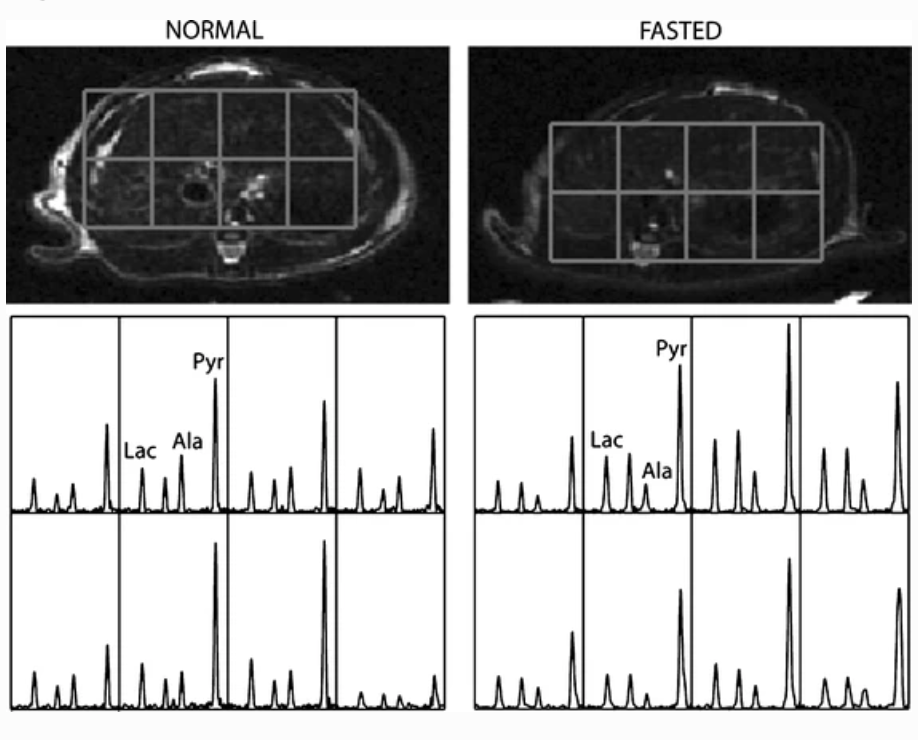
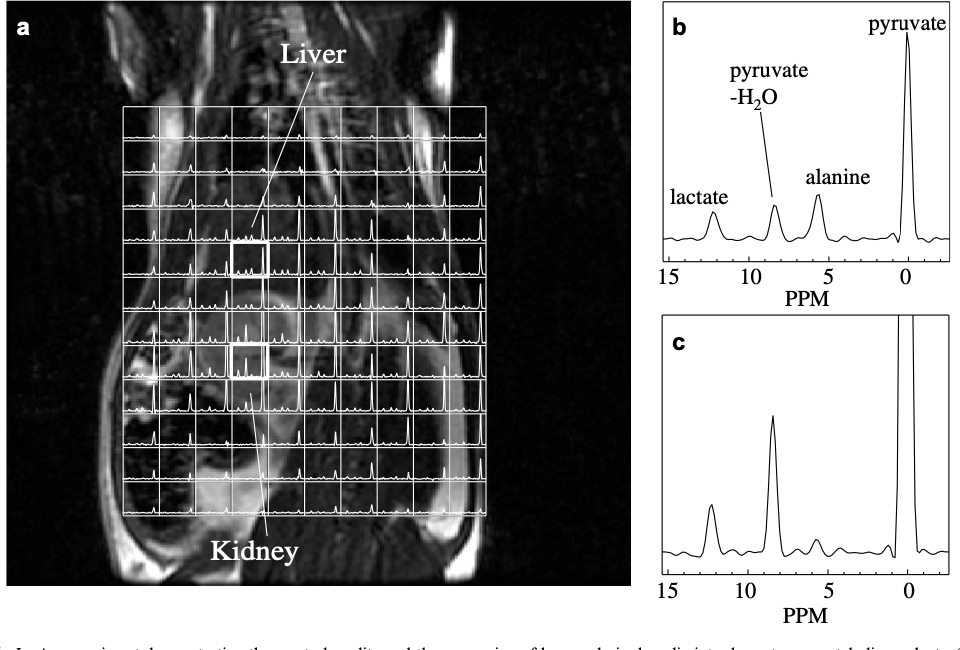
In Vivo Carbon-13 Dynamic MRS and MRSI of Normal and Fasted Rat Liver with Hyperpolarized 13C-Pyruvate
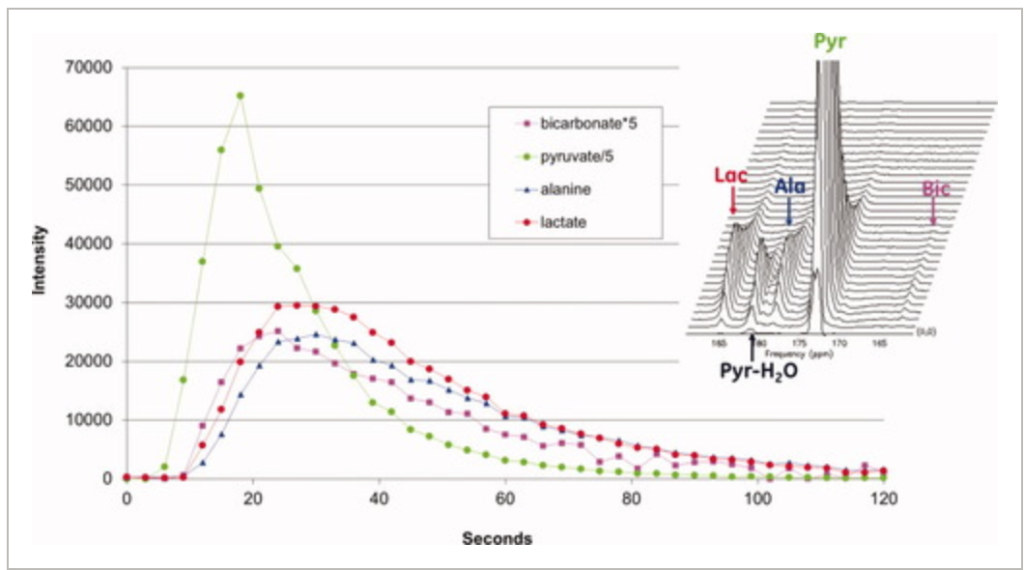
The use of in vivo 13C nuclear magnetic resonance spectroscopy in probing metabolic pathways to study normal metabolism and characterize disease physiology has been limited by its low sensitivity. However, recent technological advances have enabled greater than 50,000-fold enhancement of liquid-state polarization of metabolically active 13C substrates, allowing for rapid assessment of 13C metabolism in vivo. The present study applied hyperpolarized 13C magnetic resonance spectroscopy to the investigation of liver metabolism, demonstrating for the first time the feasibility of applying this technology to detect differences in liver metabolic states. [1-13C]pyruvate was hyperpolarized with a dynamic nuclear polarization instrument and injected into normal and fasted rats. The uptake of pyruvate and its conversion to the metabolic products lactate and alanine were observed with slice-localized dynamic magnetic resonance spectroscopy and 3D magnetic resonance spectroscopic imaging (3D-MRSI). Significant differences in lactate to alanine ratio (P < 0.01) between normal and fasted rat liver slice dynamic spectra were observed. 3D-MRSI localized to the fasted livers demonstrated significantly decreased 13C-alanine levels (P < 0.01) compared to normal. This study presents the initial demonstration of characterizing metabolic state differences in the liver with hyperpolarized 13C spectroscopy and shows the ability to detect physiological perturbations in alanine aminotransferase activity, which is an encouraging result for future liver disease investigations with hyperpolarized magnetic resonance technology.
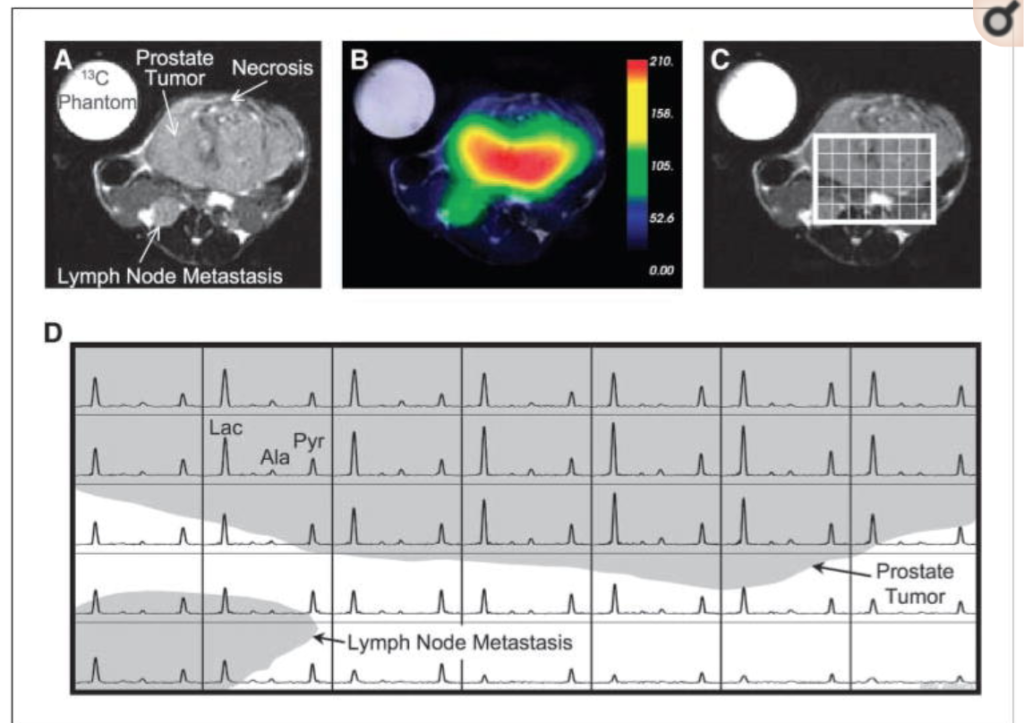
Hyperpolarized 13C Lactate, Pyruvate, and Alanine: Noninvasive Biomarkers for Prostate Cancer Detection and Grading
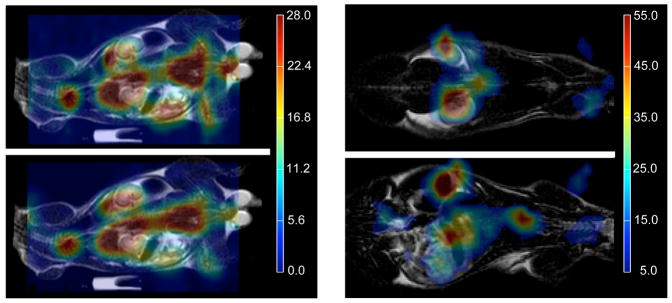
An extraordinary new technique using hyperpolarized 13C-labeled pyruvate and taking advantage of increased glycolysis in cancer has the potential to improve the way magnetic resonance imaging is used for detection and characterization of prostate cancer. The aim of this study was to quantify, for the first time, differences in hyperpolarized [1-13C] pyruvate and its metabolic products between the various histologic grades of prostate cancer using the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Fast spectroscopic imaging techniques were used to image lactate, alanine, and total hyperpolarized carbon (THC = lactate + pyruvate + alanine) from the entire abdomen of normal mice and TRAMP mice with low- and high-grade prostate tumors in 14 s. Within 1 week, the mice were dissected and the tumors were histologically analyzed. Hyperpolarized lactate SNR levels significantly increased (P < 0.05) with cancer development and progression (41 ± 11, 74 ± 17, and 154 ± 24 in normal prostates, low-grade primary tumors, and high-grade primary tumors, respectively) and had a correlation coefficient of 0.95 with the histologic grade. In addition, there was minimal overlap in the lactate levels between the three groups with only one of the seven normal prostates overlapping with the low-grade primary tumors. The amount of THC, a possible measure of substrate uptake, and hyperpolarized alanine also increased with tumor grade but showed more overlap between the groups. In summary, elevated hyperpolarized lactate and potentially THC and alanine are noninvasive biomarkers of prostate cancer presence and histologic grade that could be used in future three-dimensional 13C spectroscopic imaging studies of prostate cancer patients
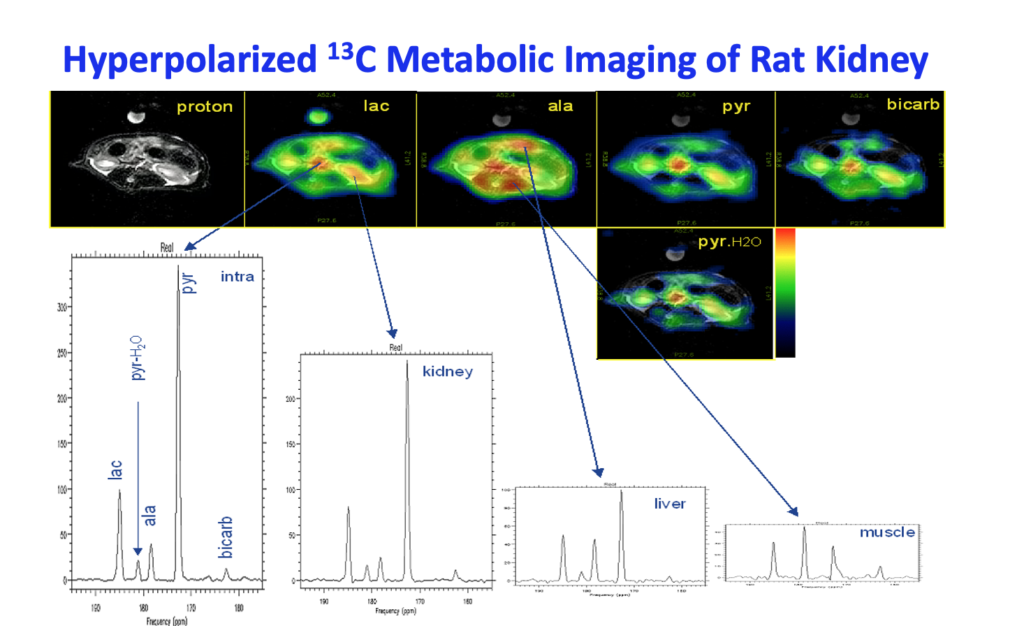
In Vivo 13Carbon Metabolic Imaging at 3T With Hyperpolarized 13C-1-Pyruvate
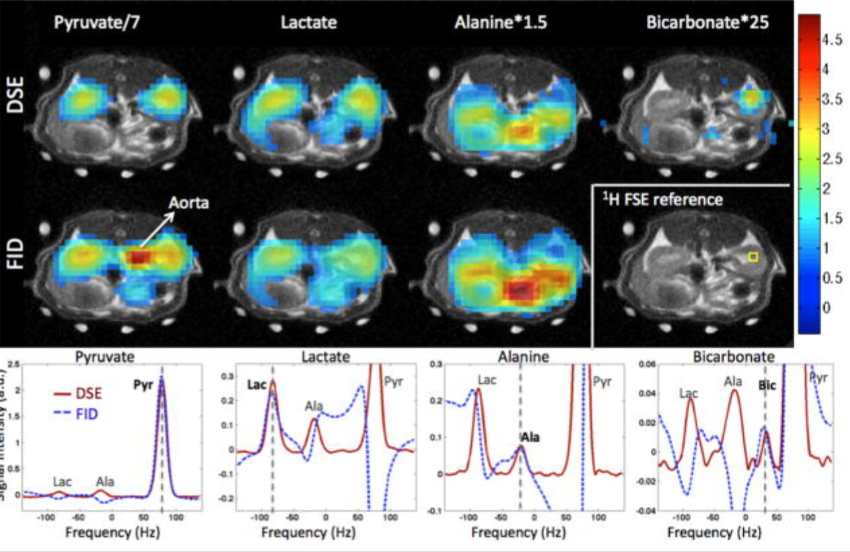
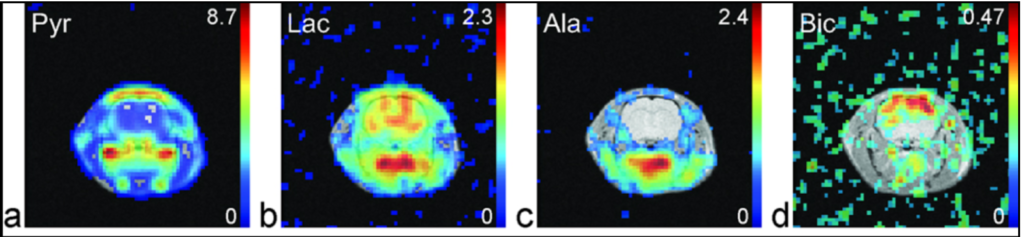
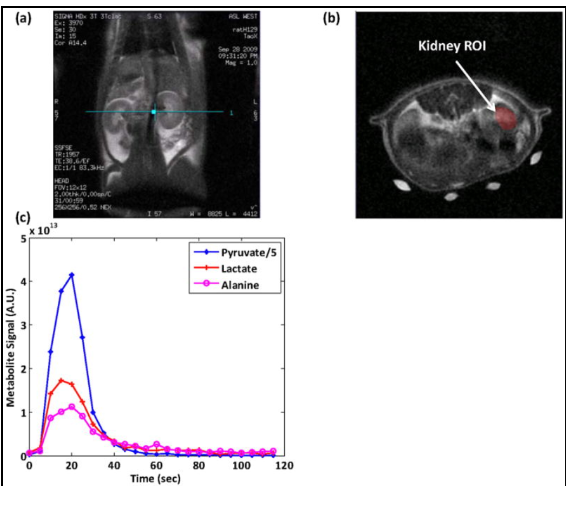
We present for the first time dynamic spectra and spectroscopic images acquired in normal rats at 3T following the injection of (13)C-1-pyruvate that was hyperpolarized by the dynamic nuclear polarization (DNP) method. Spectroscopic sampling was optimized for signal-to-noise ratio (SNR) and for spectral resolution of (13)C-1-pyruvate and its metabolic products (13)C-1-alanine, (13)C-1-lactate, and (13)C-bicarbonate. Dynamic spectra in rats were collected with a temporal resolution of 3 s from a 90-mm axial slab using a dual (1)H-(13)C quadrature birdcage coil to observe the combined effects of metabolism, flow, and T(1) relaxation. In separate experiments, spectroscopic imaging data were obtained during a 17-s acquisition of a 20-mm axial slice centered on the rat kidney region to provide information on the spatial distribution of the metabolites. Conversion of pyruvate to lactate, alanine, and bicarbonate occurred within a minute of injection. Alanine was observed primarily in skeletal muscle and liver, while pyruvate, lactate, and bicarbonate concentrations were relatively high in the vasculature and kidneys. In contrast to earlier work at 1.5 T, bicarbonate was routinely observed in skeletal muscle as well as the kidney and vasculature
Double spin-echo sequence for rapid spectroscopic imaging of hyperpolarized 13C
Dynamic nuclear polarization of metabolically active compounds labeled with 13C has been introduced as a means for imaging metabolic processes in vivo. To differentiate between the injected compound and the various metabolic products, an imaging technique capable of separating the different chemical-shift species must be used. In this paper, the design and testing of a pulse sequence for rapid magnetic resonance spectroscopic imaging (MRSI) of hyperpolarized 13C is presented. The pulse sequence consists of a small-tip excitation followed by a double spin echo using adiabatic refocusing pulses and a ‘‘flyback’’ echo-planar readout gradient. Key elements of the sequence are insensitivity to calibration of the transmit gain, the formation of a spin echo giving high-quality spectral information, and a small effective tip angle that preserves the magnetization for a sufficient duration. Experiments in vivo showed three-dimensional coverage with excellent spectral quality and SNR.
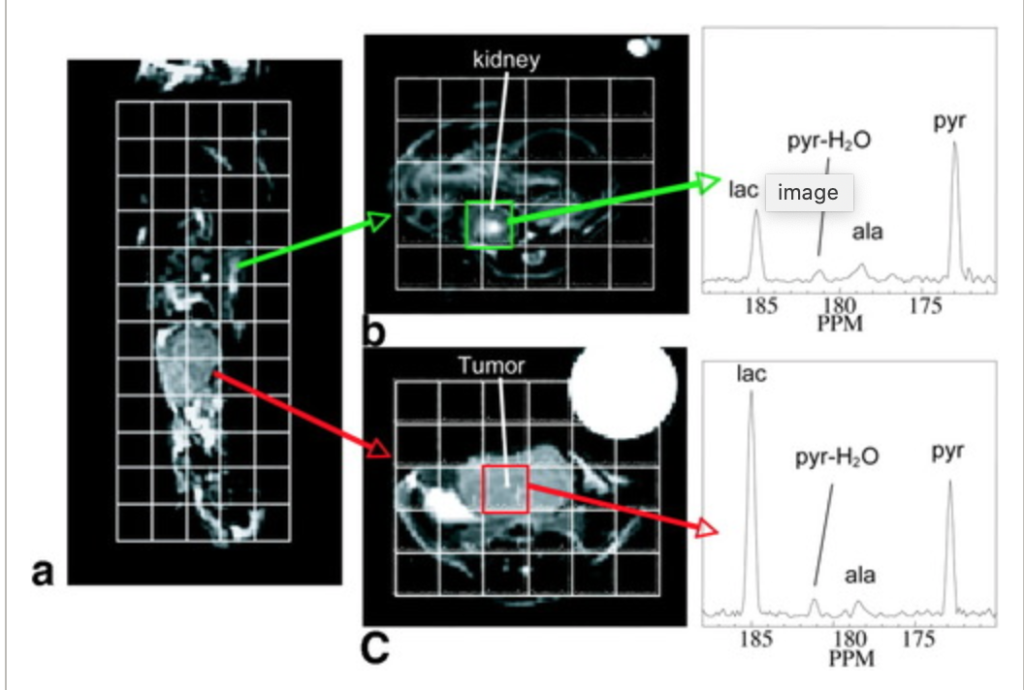
Hyperpolarized C‐13 spectroscopic imaging of the TRAMP mouse at 3T—Initial experience
The transgenic adenocarcinoma of mouse prostate (TRAMP) mouse is a well‐studied murine model of prostate cancer with histopathology and disease progression that mimic the human disease. To investigate differences in cellular bioenergetics between normal prostate epithelial cells and prostate tumor cells, in vivo MR spectroscopic (MRS) studies with non‐proton nuclei, such as 13C, in the TRAMP model would be extremely useful. The recent development of a method for retaining dynamic nuclear polarization (DNP) in solution permits high signal‐to‐noise ratio (SNR) 13C MRI or MRSI data to be obtained following injection of a hyperpolarized 13C agent. In this transgenic mouse study, this method was applied using a double spin‐echo (DSE) pulse sequence with a small‐tip‐angle excitation RF pulse, hyperbolic‐secant refocusing pulses, and a flyback echo‐planar readout trajectory for fast (10–14 s) MRSI of 13C pyruvate (pyr) and its metabolic products at 0.135 cm3 nominal spatial resolution. Elevated 13C lactate (lac) was observed in both primary and metastatic tumors, demonstrating the feasibility of studying cellular bioenergetics in vivo with DNP hyperpolarized 13C MRSI.
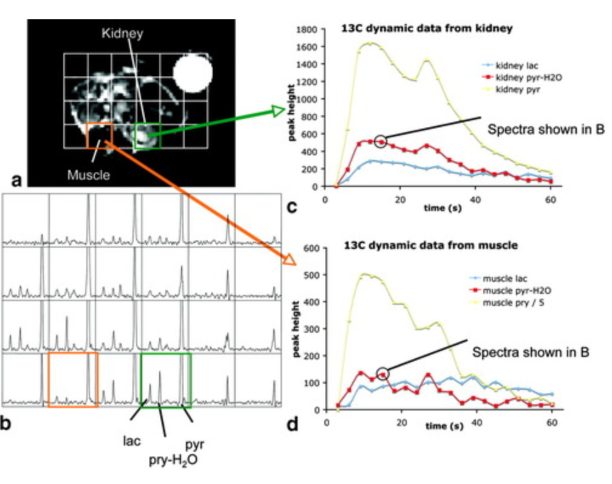
Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-Initial experience Chen AP et al. Magn Reson Med. 2007;58(6):1099-1106.
The purpose of this study was to combine a three-dimensional NMR-compatible bioreactor with hyperpolarized 13C NMR spectroscopy in order to probe cellular metabolism in real time. JM1 (immortalized rat hepatoma) cells were cultured in a NMR-compatible fluidized bioreactor. 31P spectra were acquired before and after each injection of hyperpolarized pyruvate and subsequent 13C spectroscopy. 1H and two-dimensional 1H-1H-total correlation spectroscopy spectra were acquired from extracts of cells grown in order to determine 13C fractional enrichment and distribution of 13C label. Flux measurements of pyruvate through lactate dehydrogenase and alanine aminotransferase in the bioreactor system were reproducible in the same bioreactor, but were not significantly different over the course of 2 days. Although this preliminary study involved immortalized cells, this combination of technologies can be extended to the real-time metabolic exploration of primary benign and cancerous cells and tissues prior to and after therapy.
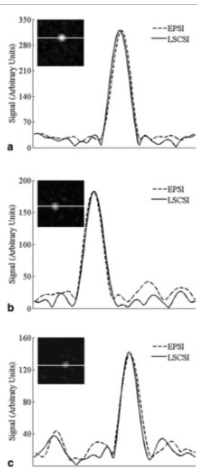
Least-squares chemical shift seperation for (13)C metabolic imaging.
Reeder SB et al. J Magn Reson Imaging. 2007;26(4):1145-1152.
The purpose of this experiment is to describe a new least-squares chemical shift (LSCSI) method for separation of chemical species with widely spaced peaks in a sparse spectrum. This method is applied to imaging of (13)C-labeled pyruvate and its metabolites alanine, pyruvate, and lactate. The least-squares method was utilized for separation of signal from the three metabolites, facilitating tremendous reductions in the amount of data required to decompose different chemical species. Results included enriched (13)C phantom imaging at 3.0T, which showed excellent metabolite separation, similar to echo planar spectroscopic imaging (EPSI), but used 1/16th as much data. Therefore, this approach may be advantageous for in vivo hyperpolarized (13)C metabolic applications for reduced scan time compared with EPSI.
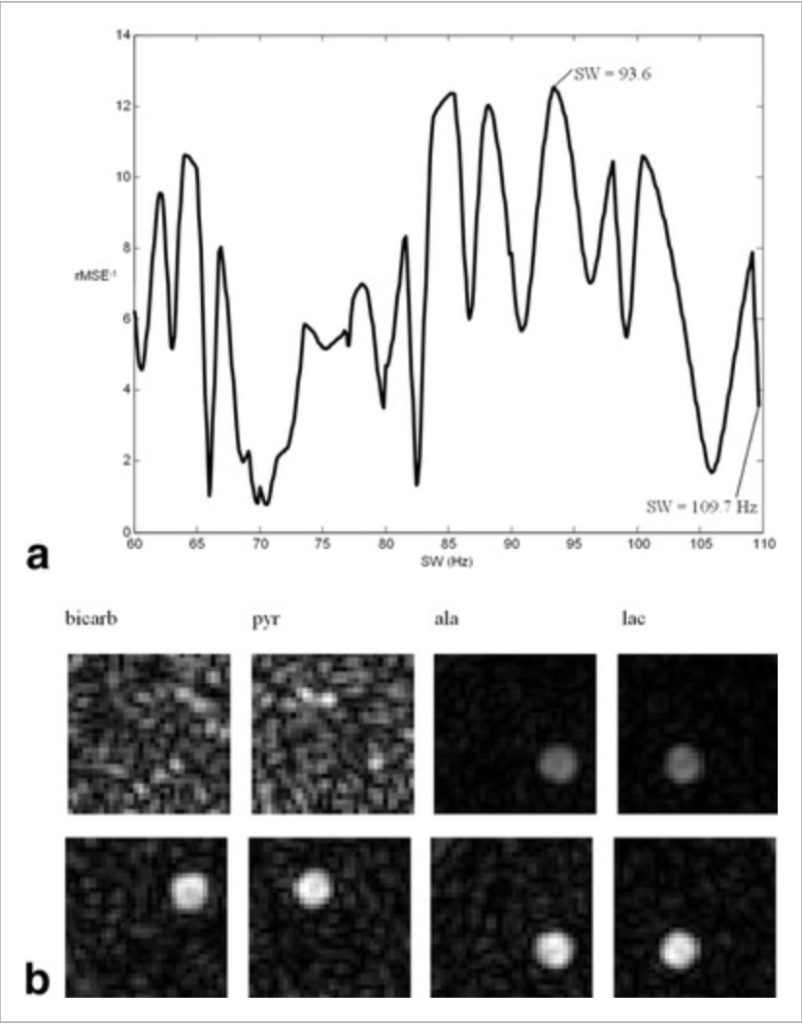
Levin YS et al. Magn Reson Med. 2007;58(2):245-252
An optimization and reconstruction algorithm has been developed for rapid metabolic imaging in the context of hyperpolarized (13)C. The algorithm uses a priori knowledge of resonance frequencies, J-coupling constants, and T(2)* values to enable acquisition of high-quality metabolic images. By performing the reconstruction in k-space, the algorithm also allows the inclusion of the effect of chemical shift evolution during the readout period. Single-interleaf multiecho spiral chemical shift imaging (spCSI) is analyzed in detail for the use of the new reconstruction and optimization algorithm. Upon reconstruction using the k-space-based least-squares technique, metabolite ratios obtained using the spCSI method were comparable to those obtained using a reference FIDCSI acquisition.

In Vivio 13 Carbon metabolic Imaging at 3T with hyperpolarized 13C-1-pyruvate
Kohler SJ et al. Magn Reson Med. 2007;58(1):65-69.
Dynamic spectra and spectroscopic images were acquired in normal rats at 3T following the injection of (13)C-1-pyruvate that was hyperpolarized by the dynamic nuclear polarization (DNP) method. Spectroscopic sampling was optimized for spectral resolution of (13)C-1-pyruvate and its metabolic products. In the experiment, combined effects of metabolism and T(1) relaxation were observed through a dynamic spectra using a temporal resolution of 3s from a 90-mm axial slab. In separate experiments, spectroscopic imaging was obtained through a 17-s acquisition of a 20-mm axial slice centered on the rat kidney region. Results revealed that conversion of pyruvate to lactate, alanine, and bicarbonate occurred rapidly, and alanine was observed primarily in skeletal muscle and liver, while pyruvate, lactate, and bicarbonate concentrations were relatively high in the vasculature and kidneys.
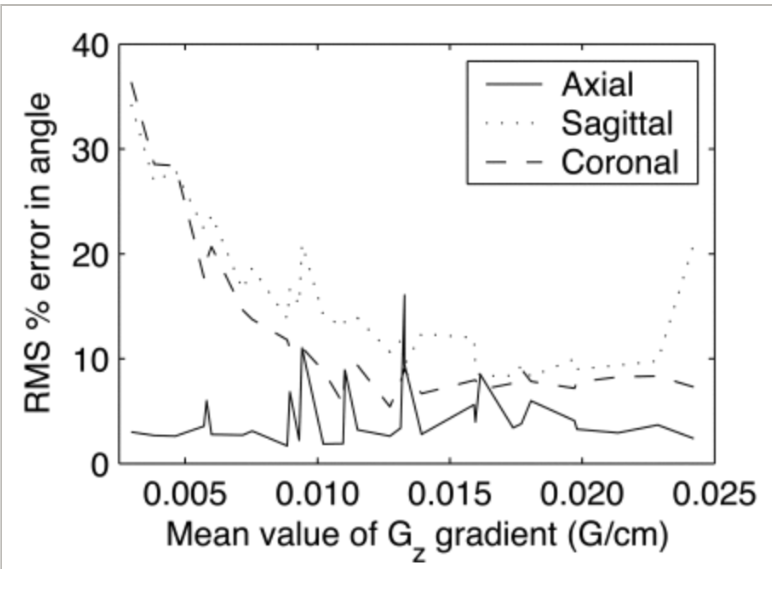
Optimizing spherical navigator echoes for three-dimensional rigid-body motion detection.
Petrie DW et al. Magn Reson Med. 2005;53(5):1080-1087
Spherical navigator (SNAV) echoes show promise in correcting for 3D rigid-body motion. In this paper, several important parameters in the design and performance of the SNAV technique are discussed, including a novel sampling strategy, the optimal k-space radius, and the execution of the SNAV trajectory. A variable-sampling density (VSD) SNAV trajectory was presented as well. To ensure that the VSD SNAV trajectory was properly executed, gradient waveforms were verified using a self-encoding technique. The results of this study show that the best accuracy was attained at k-space radii of 1.4 and 1.6 cm(-1), with 2400 to 4000 samples acquired over the sphere.


